Complement Factor H is needed to protect retinal pigment epithelium cells against oxidative stress
The EYE-RISK project investigates the interplay between genetic and lifestyle factors, that increase a person’s risk for AMD. In a study recently published in Scientific Reports Armento et al. analyzed the impact of one of the main genetic AMD risk variants, the Y402H polymorphism in the complement factor H gene (CFH). The EYE-RISK researchers could show for the first time, that factor H (FH) produced by retinal pigment epithelium (RPE) cells is essential for RPE cell metabolism and homeostasis and protects RPE cells from oxidative stress. Their results highlight a novel role for FH in AMD pathogenesis and indicate, that RPE cells within the retina might be perturbed by the CFH risk variant, especially when the genetic risk is accompanied by increased oxidative stress, which occurs naturally with age and from smoking or unhealthy diet. Possible mechanisms and their impact within the retina will be investigated in more detail in further studies.
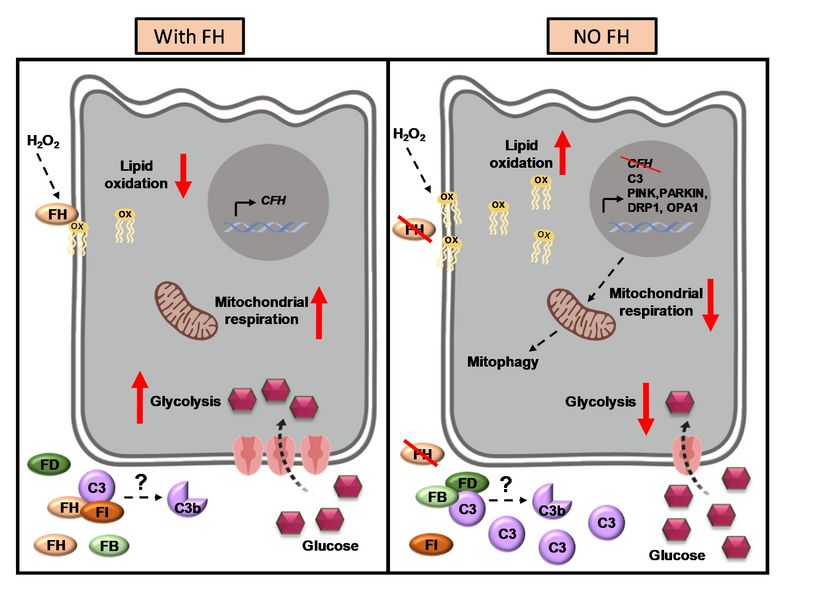
To investigate the impact of endogenous FH loss on RPE cell balance, researchers silenced CFH in a human RPE cell line. FH reduction led to accumulation of complement factor C3, at both RNA and protein level and increased RPE vulnerability toward oxidative stress. Mild hydrogen-peroxide exposure in combination with CFH knock-down led to a reduction of glycolysis and mitochondrial respiration, paralleled by an increase in lipid peroxidation, which is a key aspect of AMD pathogenesis. In parallel, cell viability was decreased. The perturbations of energy metabolism were accompanied by transcriptional deregulation of several glucose metabolism genes as well as genes modulating mitochondrial stability.