Long-term zinc supplementation identifies key regulatory pathways involved in slowing the progression to end-stage AMD
One of the main aims of the EYE-RISK project was to identify key molecular drivers and biological pathways for the development and progression of age-related macular degeneration (AMD). Given the complexity of AMD, information like this is critical to understand the disease process fully and for the development of new intervention strategies.
Dietary intake of zinc, included in the nutritional supplements AREDS and AREDS2, has shown promise in delaying the progression of AMD. In their recent open-access publication in Nutrients, Emri et al. analyzed the potential molecular mechanisms and identified specific regulatory pathways that might be involved in mediating the positive effects of long‐term zinc supplementation in AMD.
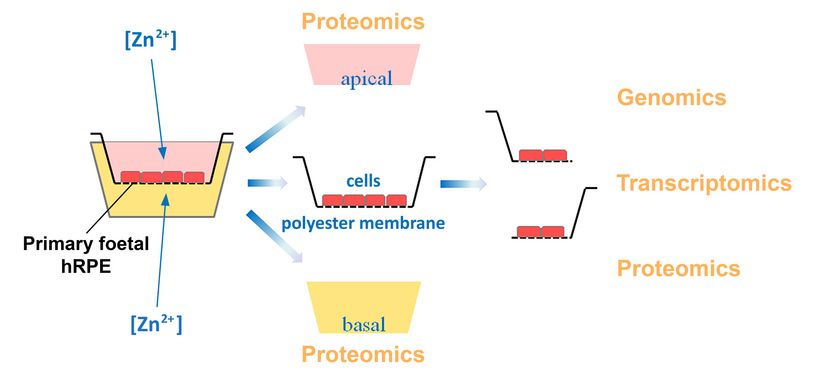
Emri et al. assessed the complex molecular changes induced by zinc supplementation in primary human retinal pigment epithelial (RPE) cells. In this study, the authors combined genetic, transcriptome, proteome, and secretome analysis of RPE cells in long-term culture. These cells recapitulated many in vivo characteristics of RPE cells in the living human eyes. The results revealed that RPE cell adhesion/polarity, extracellular matrix organization, protein processing/transport, and oxidative stress response were the most significantly affected pathways when zinc treatment was applied. The authors also reported, for the first time, that zinc supplementation could elicit a role similar to that of TGFB1. The work that was led by Queen’s University Belfast in collaboration with the University of Tubingen, I2O at Roche Pharma, and Radboud University Nijmegen, is a significant step towards understanding how zinc supplementation in the AREDS trials resulted in slowing the progression to end-stage AMD.

